Why is hydration so important for pregnant and postpartum women in particular?
Your hydration needs increase during the pregnancy and breastfeeding periods.
Let’s chat hydration in pregnancy, postpartum and breastfeeding women and how it works.

For Pregnant Women
DID YOU KNOW?Blood plasma volume increases by approximately 6% in the first trimester, 18-29% between 14-27 weeks and 40-50% by the end of the third trimester. That’s almost a one and a half times increase in blood volume!
YOU NEED HYDRATION TO:- Provide the water needed to form the amniotic fluid that surrounds and cushions your baby - hydration directly affects the amniotic fluid
- Produce the additional blood supply to your circulation and support your expanding plasma volume
- Produce the additional blood supply needed to support baby’s circulation
- Improve your digestion and aid in absorption of water-soluble vitamins
- Carry the additional (and required) nutrients from your diet
- Eliminating waste products
- Maintain your body temperature
- Ensure there is enough reserve to tolerate blood loss during delivery
- Urine (filtered through the kidneys): the kidneys become slightly larger in pregnancy with an increase blood flow rate and hence increase urine production during pregnancy.
- Sweat (through your skin): increased sweating during pregnancy
- Breathing (through the respiratory system): there is an increased respiratory rate in pregnancy and hence increase loss of water
- Bowel motions (through the digestive system): increased nutrient and energy requirements for pregnant women and lactating women leads to increased food intake. Water is the carrier of these nutrients

For Breastfeeding women
DID YOU KNOW?Breastfeeding women need extra nutrients and this is because the body is working harder to make breastmilk full of nutrients for your baby.
REPLACE LOST FLUIDSLactating women need to replace fluid lost in breastmilk to maintain hydration. Water accounts for 87% of breastmilk. Breastfeeding women produce (on average) 780ml per day for the first 6 months which is equivalent to 700ml of water. This is why a breastfeeding woman needs to drink 700ml above the above the normal daily requirements to maintain hydration
How do electrolytes work in helping hydration?
The electrolytes in HydroBump aid in hydration and enhances the water absorption process in the body by utilising the bodies natural cell mechanisms – hence a hydration booster!
Water is the foundation of life and the most critical nutrient of the body. Maintaining an optimal level of water in the body, especially during pregnancy and the postpartum period, is essential as too little or too much can lead to less-than-optimal functioning.
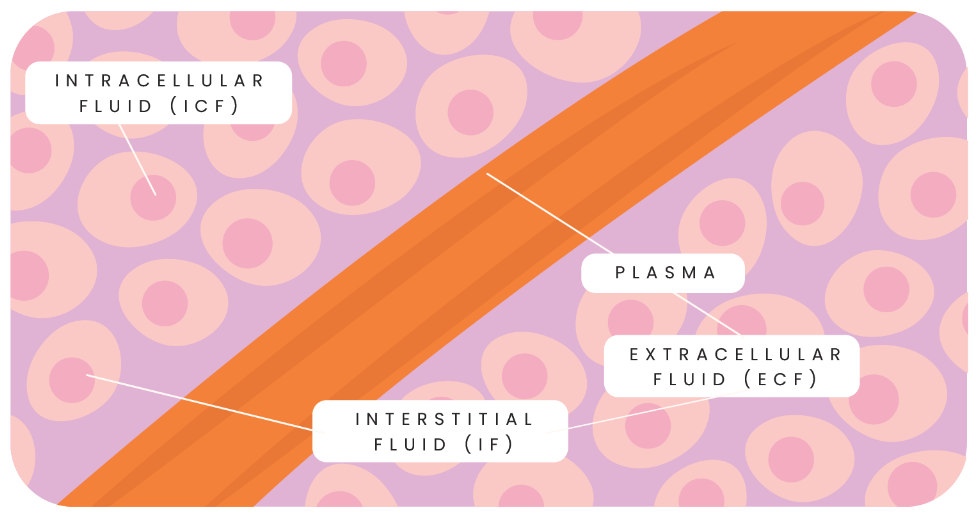
Basically, water is distributed in two places in the body: inside cells and outside cells. Within this water, there are things called solutes (such as electrolytes) and these work together to allow for water to move inside and outside cells as needed. Water moves freely in and out of cells through a process called osmosis – where it moves to areas of higher solute concentration.
Here’s an example of how electrolytes works (cue high school human biology lessons!).


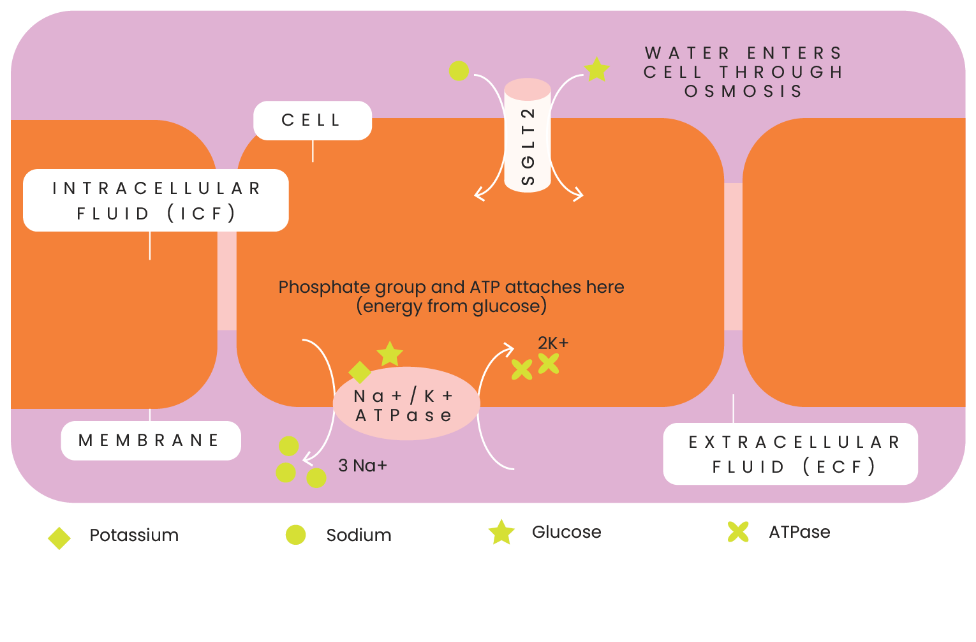
The Sodium Potassium Pump
In cells, there’s a protein called the Sodium Potassium Pump (Na+K+ ATPase). It’s main function is to pump three sodium molecules (positively charged) out of the cells and two potassium molecules (positively charged) into the cells which helps to stabilise the cells outer layer (the membrane). This pump requires energy to function (also known as ATP – adenoseine-tri-phophate - hence why our formulation has phosphate) and this energy comes from glucose (which is why our formulation is LOW sugar and not zero sugar). The ATP is broken down and transfers a chemical group known as a phosphate group to the pump. This essentially kickstarts the pump to start working (known as a conformational change) and this kicks out the 3 molecules of sodium and brings in the 2 molecules of potassium hence maintaining strict electrochemical gradients needed for cell functioning. How does this relate to water and hydration? Well, all these reactions we’ve talked about take place in water! So if we don’t have adequate hydration, it can upset this very delicate balance required to maintain life!
Another example of the importance of electrolytes in maintaining cell functionality is in the small intestine. There is another protein called the Sodium-Glucose Co-transporter. (SGLT).
There’s a mechanism known as the sodium/glucose co-transport system found in the small intestine. When sodium gets to the small intestine, it’s pulled into the cell by the SGLT, along with glucose, into the cell. So remember how the Sodium Potassium Pump (NA+K+ ATPase) we spoke about earlier was kicking out 3 molecules of sodium? So the cell has slightly less sodium inside than outside the cell so the SGLT then tries to correct this by pulling in a sodium molecule WITH a glucose molecule. Water then follows the glucose by the process of osmosis hence hydrating the cell.

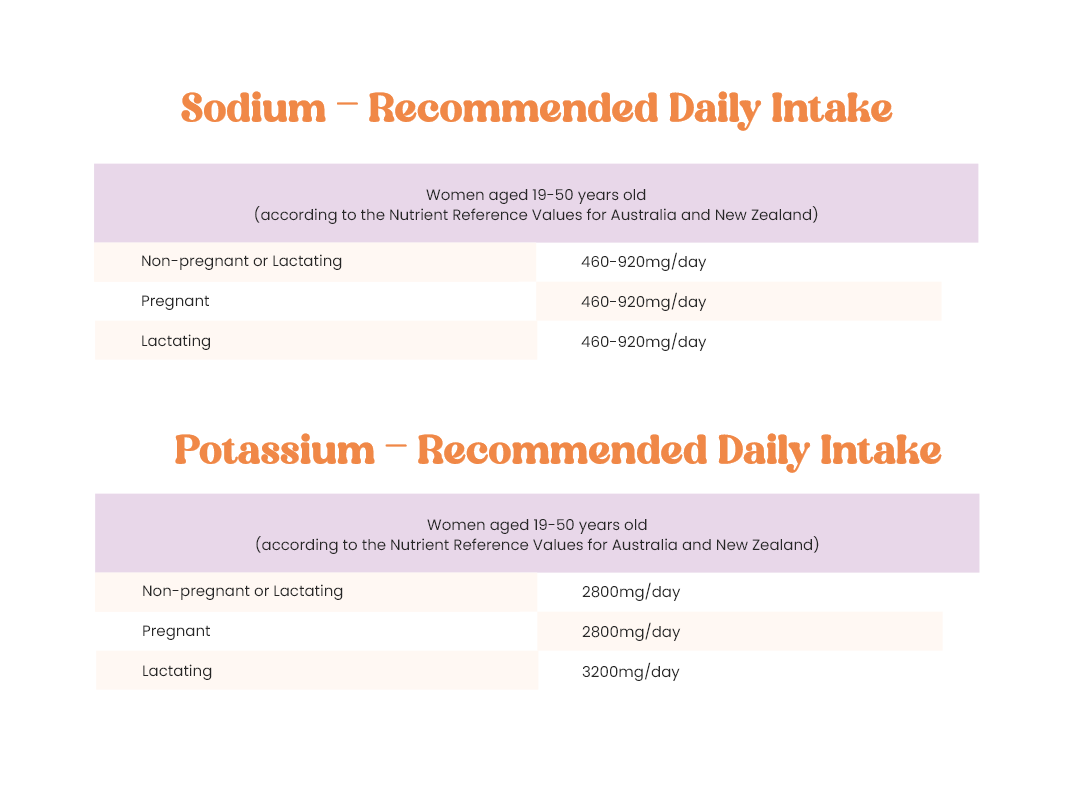


What about the Chloride component of the formulation?


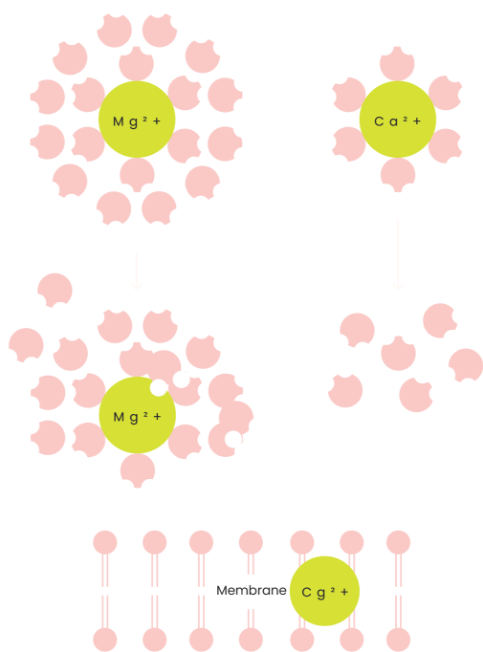
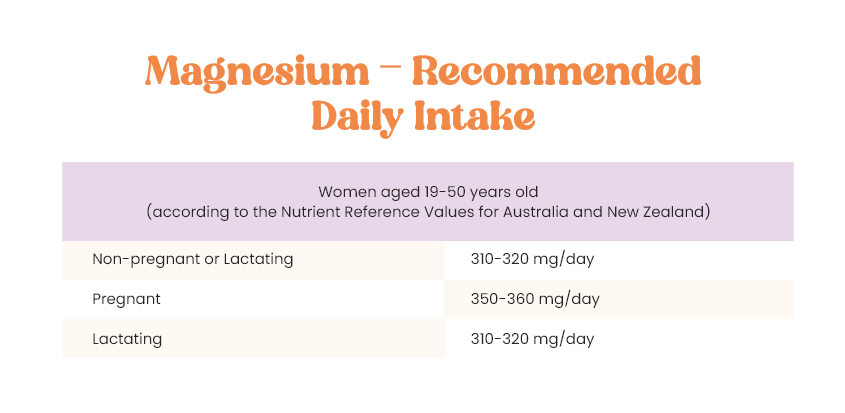
What about magnesium?
Magnesium has an affinity with water molecules (meaning it likes to bond with them) and allows the water to become more “hydrating” for the cells and has a high hydration energy due to its double layer of water molecules. So basically magnesium allows more water to get into the cells and aids in holding the water inside our cells keeping the cell hydrated for normal functioning. If a cell loses too much magnesium, there is an imbalance that causes the cells membrane to become “leaky” and hence prevents normal hydration. And hence why we added magnesium!
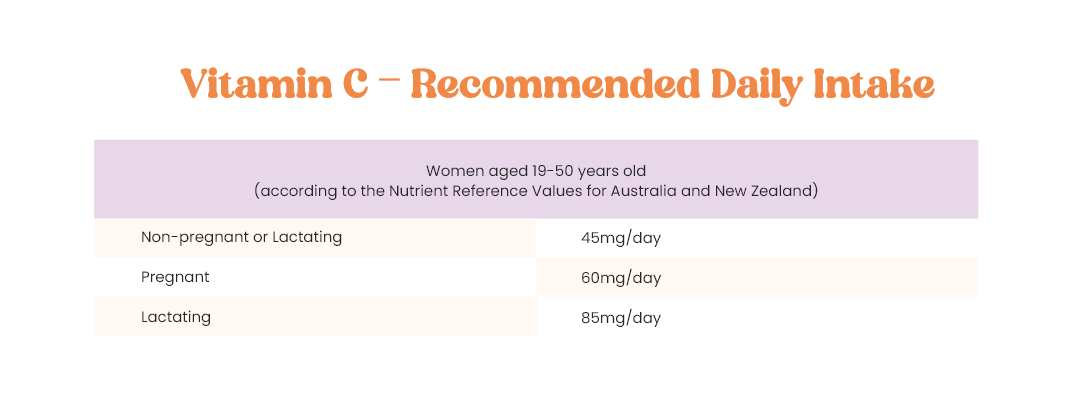
What about vitamin C?
There are numerous benefits of vitamin C and even more so during pregnancy and breastfeeding as you need more mg/day! Vitamin C contributes to iron absorption from food, necessary for normal connective tissue structure and function, cell protection from free radicals, normal collagen formation for skin, cartilage and bone, immune system function and so much more.
 THESE PROCESSES ARE ALL INTERLINKED, CRITICAL FOR SUSTAINING LIFE AND HIGHLY SENSITIVE
THESE PROCESSES ARE ALL INTERLINKED, CRITICAL FOR SUSTAINING LIFE AND HIGHLY SENSITIVE
Here’s where we come in!
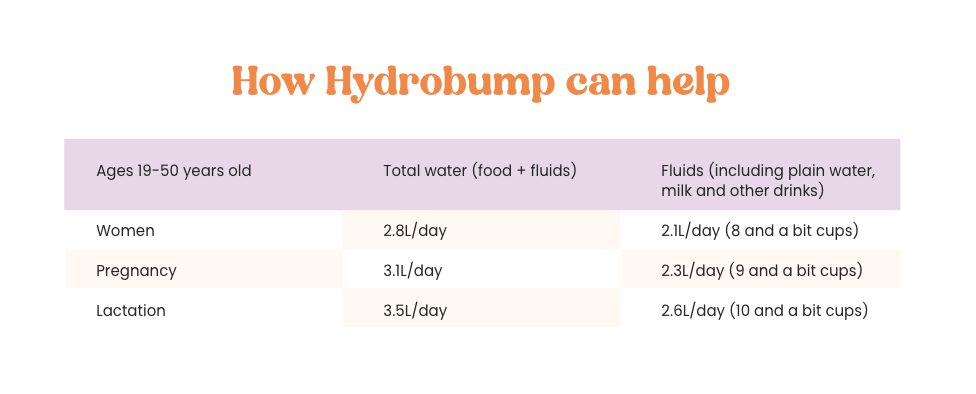
References:
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3676933/
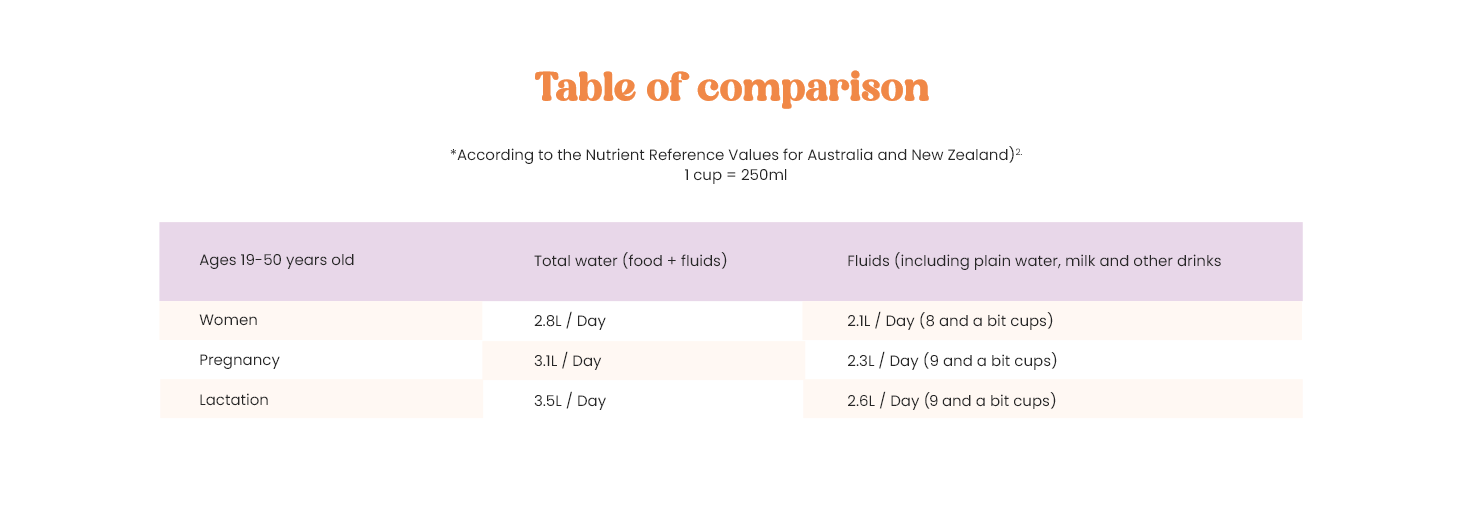
- https://www.eatforhealth.gov.au/nutrient-reference-values/nutrients/water
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1595116/#: ~:text=Pregnancy%20Needs,glasses%20of%20water%20each%20day.
- https://bmcpregnancychildbirth.biomedcentral.com/articles/10.1186/s12884-019-2301-z#:~:text=On%20average%2C%20the%20TWI%20of,the%20adequate%20intake%20(AI).
- https://bmcpregnancychildbirth.biomedcentral.com/articles/10.1186/s12884-019-2619-6
- https://bmcpregnancychildbirth.biomedcentral.com/articles/10.1186/s12884-020-2765-x


We donate with every purchase
Supporting women supporting women.
We partner with Handup Congo who respond to community requests by providing the tools and training to help Congolese communities generate income to improve education, health and wellbeing.
With every purchase, we donate to their amazing cause.
 LEARN MORE
LEARN MORE
Our products

We’re here to help YOU!
Let’s face it. Getting pregnant, pregnancy, postpartum… it’s not easy!
We’ve created a community of strong, empowered, beautiful and likeminded women so you never have to feel alone in your journey.
Lets chat everything from your experience to bettering your hydration and everything in between!